生体分子化学研究室(野瀬研究室)のページ
reSEARCH
Contents of the research
Our laboratory mainly studies three subjects that are focus areas in peptide and protein chemistry research:(1) Structure-function relationship of elastin-derived peptides
(2) Determination and discovery of receptor-binding molecules (in particular, risk assessment of endocrine disruptors using a combination of computational and biochemical techniques)
(3) Development of molecular transformations based on multicomponent systems (establishment of new organic synthesis reaction fields) : Under construction.
1. Structure-function relationship of elastin-derived peptides
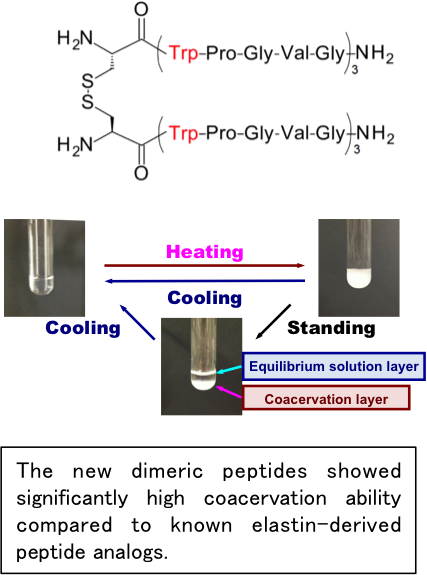 Elastin, a core protein of the elastic fibers, exhibits coacervation—a temperature-dependent reversible association/dissociation—under physiological conditions. Due to this unique characteristic, elastin and elastin-derived peptides are considered valuable as base materials for the development of various biomedical products such as skin substitutes, synthetic vascular grafts, and drug delivery systems.
Elastin, a core protein of the elastic fibers, exhibits coacervation—a temperature-dependent reversible association/dissociation—under physiological conditions. Due to this unique characteristic, elastin and elastin-derived peptides are considered valuable as base materials for the development of various biomedical products such as skin substitutes, synthetic vascular grafts, and drug delivery systems.
In our recent studies, we have developed a new series of elastin-derived
peptide dimers: (Cys-(Phe-Pro-Gly-Val-Gly)5)2 and (Cys-(Trp-Pro-Gly-Val-Gly)3)2. These dimers possess a high coacervation potential. Impressively, these novel dimeric peptides demonstrated coacervation at significantly lower concentrations and temperatures compared to commonly used elastin-derived peptide analogs. This suggests that the dimerization process enhances the peptides' coacervation ability. Building on these findings, we are in the process of developing more potent elastin-derived peptide analogs that combine both biological and chemical functionalities.
.
2. Determination and discovery of receptor-binding molecules
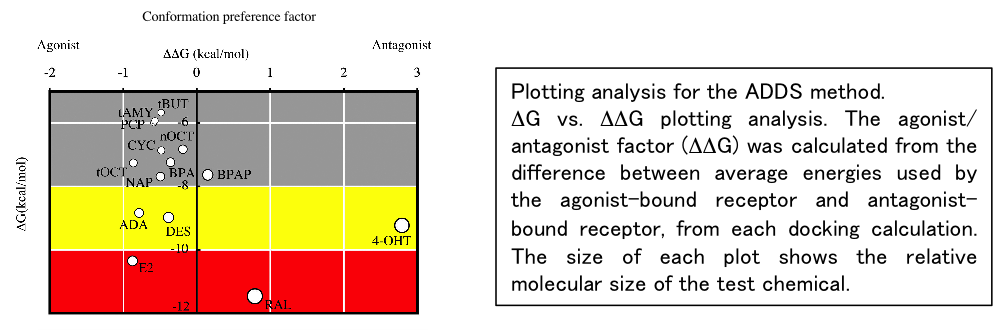
We conducted risk assessment studies on endocrine-disrupting chemicals (EDCs) using a blend of computational and biochemical techniques. Nuclear receptors (NRs), which act as central transcription factors in biological processes, are primary targets of EDCs. Identifying chemicals that can bind to the ligand-binding domain (LBD) of NRs is crucial as these chemicals can cause significant disruptions in the endocrine system.
To address this, we introduced a novel screening approach, leveraging in silico docking calculations. Utilizing the crystal structures of both agonist-bound and antagonist-bound LBDs as templates, we estimated binding energies to differentiate between agonist and antagonist binding. This method, termed agonist/antagonist differential-docking screening (AADS), not only predicts the binding capability but also the agonist/antagonist function of chemicals for the target NRs.
3. Development of molecular transformations based on multicomponent systems
(establishment of new organic synthesis reaction fields)
: Under construction..
Laboratory of Biomolecular Chemistry
〒819-0395
744 Motooka Nishi-ku Fukuoka 819-0395 Japan
TEL 092-802-6025